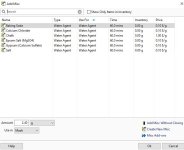
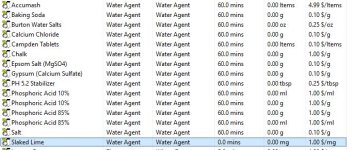
Hi, when adding water salts to my RO water profile, slaked lime, among others, isn't a choice. I would think all water agents would be available? I tried the Add Salts button but that doesn't seem to do anything. I attached a picture of what water agents I"m able to add to my water profile, and the ones that are truly available in the Misc. Ingredients section.
Thanks,
Mark
Thanks,
Mark