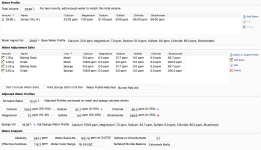
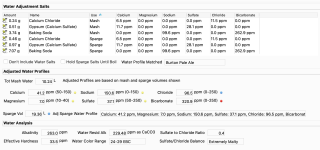
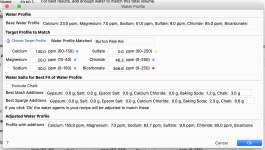
I'm trying to match "Burton Pale Ale" by using the Water tab and "Match a Target Profile".
The Adjusted Water Profiles section shows my "Bicarbonate" as 346.6ppm (range of 0-250 being optimal?) and has a little red icon next to it, presumably indicating that this isn't advisable.
Why would this be?
The Adjusted Water Profiles section shows my "Bicarbonate" as 346.6ppm (range of 0-250 being optimal?) and has a little red icon next to it, presumably indicating that this isn't advisable.
Why would this be?