This week I take look at hop isomerization in beer brewing and how it applies to brewers. Isomerized alpha acids in beer are the primary bittering compound used to offset the sweetness of the malt in beer.
What is Isomerization?
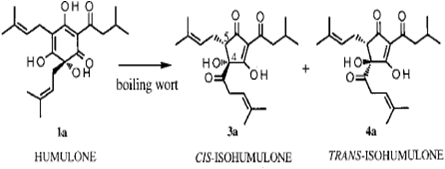
Isomerization is a process where a molecule is transformed into another molecule that contains the exact same atoms (isomers) but is arranged differently. In the case of hops, we are primarily concerned with alpha acids, also called humulones, that get transformed into isomerized alpha acids (called isohumulones) that provide the bulk of the bittering compounds in finished beer.
Alpha acids make up a percentage of dried hop cones that are harvested, dried and compressed into pellets for beer brewing. The alpha percentage is typically listed on the package of hops you purchase from your homebrew store.
Heat by boiling is the primary mechanism for isomerizing the alpha acids present in the hops. The longer you boil the hops, the larger percentage of the hops that will be isomerized. Software like BeerSmith use equations to estimate the isomerization of the hops and determine the approximate bitterness level in International Bitterness Units (IBUs) going into the fermenter. If you measure IBUs in a finished beer, the measurement done attempts to determine the concentration of isomerized alpha acids in the beer.
While isomerization happens at a fixed rate at boiling temperatures it can also take place at lower temperatures. For example at high altitude, water boils at a lower temperature resulting in lower hop isomerization (utilization) which brewers need to compensate for. Similarly hops added after the boil has ended, called whirlpool or steeped hops, will still result in some isomerization although at a much lower rate due to the reduced temperature. In fact the isomerization rate drops off rather quickly with temperature, reaching only about 10% of the equivalent boiling rate when the temperature is 70C (158 F).
Is Isomerization the Whole Story?
As I mentioned at the beginning of the article, the bulk of bitterness in beer that we use to offset sweetness from the malts used comes from isomerized alpha acids. However that’s not the whole story. The perception of bitterness is more complex than that.
For example, a high gravity or dark beer might require substantially more bitterness to achieve the same level of perceived bitterness than a low gravity beer. That’s why many of us use something called the bitterness ratio to determine the balance between maltiness and bitterness in the beer.
Beyond that the water used in brewing also affects the perception of bitterness. Something called the sulfate to chloride ratio, which is a measure of sulfate/chloride ions in your brewing water also affects how we perceive bitterness on the tongue.
Aroma oils from the hops also impact our perception of bitterness. When we taste a beer, our tongue is only doing a small portion of the work. Much of what we perceive as flavor comes from the nose as the nose, which is much more sensitive and can detect hundreds of thousands of compounds. That is why craft breweries spend many millions of dollars each year on whirlpool and dry hopping techniques designed to preserve delicate hop aroma oils.
There are also other compounds in hops that affect bitterness. Recent University research indicates that some bitterness can be imparted by dry hopping, even though that bitterness does not come from isomerized alpha acids. This is an area of ongoing research.
While isomerization is not the whole story, it is still critical for brewers to understand the basic process, and how both boil and whirlpool hopping can be optimized to take advantage of isohumulones.
Thanks for joining me on the BeerSmith Home Brewing Blog. Be sure to sign up for my newsletter or my podcast (also on itunes…and youtube for more great tips on homebrewing.
I’m learning every moment when tuning to your podcast and every guest you have invited.
I grow hops in my garden in the UK and use them for making beer in September. I’ve noticed that if I make a hop tea (e.g. for dry hopping) by just adding boiling water to a mug of fresh hops, the resulting tea is very bitter to taste. Obviously, not much chance of isomerization in this case. Even putting the hops into cold water produces a very bitter taste in the water. So the isomerization does not seem to be necessary for bitterness. The intensity of the bitterness is, of course, difficult to measure.
I recently tasted some trub in a very hoppy NEIPA I’d made. Overall the bitterness is low in the finished beer. There was some whirpool hopping and a couple of dry hop charges all to try and drive flavour and aroma rather than bitterness. Upon tasting less than a teaspoon of trub, with hop particulate throughout…my goodness, it was bitter. I now understand what people mean when they say bitterness is detected by the whole digestive system. It was a lingering bitterness that was still present more than 15 minutes later.
Does my tongue ‘isomerise’ the alpha acids in hops? I was shocked at how massive, rapid and long lasting the bitterness was.