
This week I provide an overview of water and its use in beer brewing. While water chemistry is considered by some to be an advanced topic, it makes up 90% of most beers and has a tremendous impact on your finished beer.
Why Water Matters
In blind tasting tests, changes to the water used in a beer are some of the easiest to detect. This is because water makes up 90%+ of most beers, and our senses are well tuned to sense fresh water. This is why major commercial breweries are often built around a single water source.
Understanding Your Local Water
Tap water is generally harvested from two major sources: surface water from lakes, rivers and streams and groundwater which comes from underground aquifers. Groundwater often contains a higher mineral content, but fewer organics (algae, bacteria, etc). Surface water has organics which much be treated, but fewer minerals. Further adding complexity is the fact that some municipalities switch sources several times during the year, so your tap water may actually change over time.
As a brewer, you need to understand your base water profile for your local water. This means measuring the big six water ions mentioned below. The best way to do this is either submitting your sample to a water testing lab like Ward Labs or White Labs. It is important to use a “Brewing Water Test” and not a generic water test as you want the specific information on the brewing water ions and not a measure of bacteria or other organics.
Another option is to purchase a test kit from a source such as the Smart Brew test kit or Lamotte BrewLab test kit. These test kits let you perform multiple tests and can be shared with a small group like your local club or friends, even though they have a higher price point.
Water Ions and What They Mean
Brewers are primarily concerned with six water ions when looking at a water profile. These water ions are typically listed in parts per million (ppm) though you may also see them as mg/L which is the same thing. Here are the six along with the recommended ranges to use for brewing.
- Calcium (Ca) [50-100 ppm] – Acidifies the mash and drives down the mash pH which is generally a good thing in lighter color beers. It also aids in precipitating phosphates and improving the stability of the beer. Calcium also provides some structure to the beer and is used in that role as well.
- Bicarbonate (HCO3) [0-250 ppm] or Alkalinity [0-200 ppm] – Strongly alkaline so it will raise the mash pH which is undesirable in lighter color beers. High levels also will impede the cold break and emphasize bitterness in a harsh way. The main role for these ions is in mash pH balance, and too much alkalinity can be undesirable. You can convert from bicarbonate to alkalinity using this equation: alkalinity = bicarbonate * 50 / 61.
- Sulfate (SO4) [50-250 ppm] – Suilfate enhances bitterness in beer and slightly lightens color. Its primarily role is as a counterbalance to chloride in determining the sulfate/chloride ratio which affects bitterness perception in the final beer.
- Chloride (Cl) [0-250 ppm] – Chloride will lower the bitterness perception and enhance the maltiness of the beer. Its major role, along with sulfate, is in determining the sulfate/chloride ratio which affects bitterness perception in the beer.
- Sodium (Na) [0-150 ppm] – Sodium provides enhances the sweetness and body in certain dark beers. However it is not a major player in mash pH so it is primarily used to provide some roundness in darker beers.
- Magnesium (Mg) [10-40 ppm] – Magnesium plays an important role in fermentation as it is needed by yeast, but grains in the mash do provide about 100ppm of magnesium. However, recent research indicates that calcium may actually block the yeast’s access to magnesium if it is very high. So it may be important to keep the total magnesium levels (water plus magnesium from the mash) above the calcium to enhance yeast health. Magnesium also plays a small role in mash pH, but is largely overshadowed by bicarbonate and sulfate.
I created this graphic to help you understand how the base water ions:
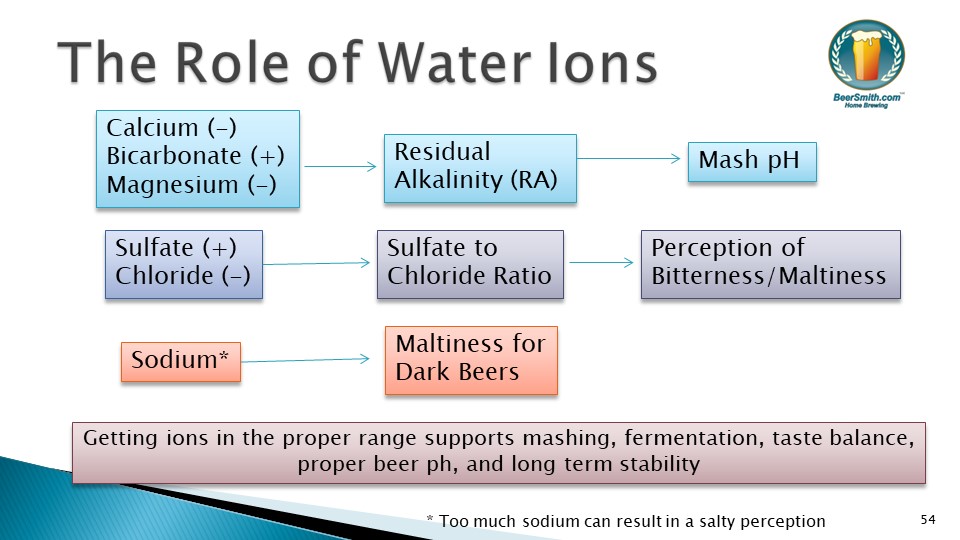
As you can see, Calcium and Bicarbonate (to a lesser degree Magnesium) are the major drivers in determining Residual Alkalinity. Residual alkalinity (RA) is a measure of how hard it is to move your mash pH, so low RA will mean you need less acid to move your mash pH while a high RA will make it harder to change the mash pH using acidity. Adjusting your mash pH to get it in the “good” range of 5.2-5.6 during the main mash conversion step is an important goal for all grain brewers.
The Sulfate to Chloride ratio can be simply calculated as the by dividing the sulfate ion number by the chloride number. It drives the perception of bitterness in the beer and can also be manipulated to make your beer taste more malty or more bitter.
Finally, sodium plays a relatively minor role in adding maltiness and roundness to some darker beer styles.
Adjusting Your Brewing Water
Water adjustments for beer brewing fall into two categories: salt additions and acid additions. Brewing salt additions are done early in the brewing process to adjust the base water profile ion content to achieve a different starting water profile. Acid additions are typically done before mashing in with the primary goal of achieving a “good” mash pH of between 5.2 and 5.6 during the main conversion step in the mash.
I will mention if you are an extract brewer, very likely the malster that created your malt extract has already done a lot of the water chemistry for you, and those minerals remain in the malt extract as a result of the concentrating process they use. Therefore using neutral or even distilled or RO water might be most appropriate if you are brewing primarily with malt extract.
For all grain brewers, brewing software like BeerSmith, make it easy to calculate your salt additions. As shown in this video you can basically add your local starting water profile to your recipe and then just select which water profile you want to match and the software will calculate the best combination of salts to add. You can do the same thing with our BeerSmith Web version.
I will mention there is an important limitation – you can’t “remove” ion content from your water profile. So if your base water profile has 100 ppm of Calcium and your target is 50 ppm of Calcium the only want to reduce that number is to dilute the base water with something like RO or distilled water. In BeerSmith you can accomplish this by (for instance) adding half of the total water volume needed in base water and half as distilled water (i.e. add two water ingredients to your ingredient list).
Similarly, you can use the software to estimate your mash Ph for a given base water and recipe in BeerSmith and then calculate how much acid is needed to achieve a given mash pH. This video shows you how to do this easily. You also might want to consider where and when to add the acid as well as when to measure your mash pH, as timing can be important to achieve your best results. This article details the best strategy for timing your acid additions for adjusting your mash pH.
I hope this summary post can help you understand the somewhat complex role water plays in our beer as well as the primary ways we adjust our water to create great beer. I urge you to read the more detailed posts linked above if you want to understand particular aspects of water profiles or water adjustment.
I hope you enjoyed this week’s article from the BeerSmith Home Brewing Blog. Please subscribe for regular weekly delivery, and don’t hesitate to retweet, link, like or mention any of my articles on social media.